As Stern, Ferraro and Mohnkern point out in “Tools for Teaching Conceptual Understanding: Designing Lessons and Assessments for Deep Learning”, the biggest pitfall that teachers face when introducing a new concept is that they are tempted to introduce it as a fact. This means it becomes a definition to be memorised, rather than a concept to be understood. To avoid this, teachers can intentionally design activities that allow students to form their own ideas about concepts through drawing patterns from examples and non-examples. This is a very powerful idea and I have applied it to the concept of open, closed and isolated systems in chemistry.
Open Systems
Carry out a neutralisation reaction between hydrochloric acid and sodium hydroxide, with a temperature probe or thermometer in the solution. When you add the solutions together, the temperature should rise. You can also get students to feel the beaker. Tell students that this is an example of an open system. Ask students to think about this example and use it write down what they think an open system is. They should come up with something like: an open system is one where energy can be exchanged with the surroundings.
So, they have one piece of the puzzle.
Now, carry out the reaction of calcium carbonate with hydrochloric acid on a mass balance. Once again, point out that this is an example of an open system. Ensure that the container that the reaction is carried out in is open. Again, place a thermometer or temperature probe in the solution. When the two substances are added together, the students should see a temperature rise (and again, can feel the container). However, this time they should also see that a gas is being produced and that they mass of the substances in the container is decreasing. Ask students to use this example to add to their musings on what an open system is (I recommend getting them to use a different coloured pen). They should come up with something along the lines of: an open system is one where energy and matter can be exchanged with the surroundings.
Closed systems
Place some liquid bromine in a gas jar (make sure this is done in a fume cupboard with the fan turned on - you’d be surprised how many people forget to turn fans on…). At this point, you could reinforce the concept of open systems and ask students why it is currently an open system. Next, add a lid on top of the gas jar. Tell students that this is now an example of a closed system. Ask students to think about this example and use it write down what they think an closed system is. They should come up with something like: a closed system is one where matter cannot be exchanged with the surroundings.
At this point, we are not finished, since students will most likely not have brought in the idea of energy.
So, next, react some Mg with HCl in a conical flask that is attached to a gas syringe. Ask students whether this fits with their understanding of what a closed system is and why. Now, get students to feel the conical flask. It should be warm. Ask students to think about what this might mean for their understanding of what a closed system is. They should come up with something like: a closed system is one where matter cannot be exchanged with the surroundings but energy can.
Isolated Systems
For this one, we need to rely more on the non-examples, since there is only one real example of an isolated system. Tell students that the universe is an example of an isolated system, whereas all the reactions that they have just seen are non-examples. You might like to give a couple of other non-examples. Ask them to use this example and the non-examples to write down what they think an isolated system is. They should come up with something like: an isolated system is one where both matter and energy cannot be exchanged with the surroundings.
Follow up
To follow this up and consolidate their understanding, I would ask student to represent all of these systems diagrammatically on a whiteboard. They should come up with something along these lines:
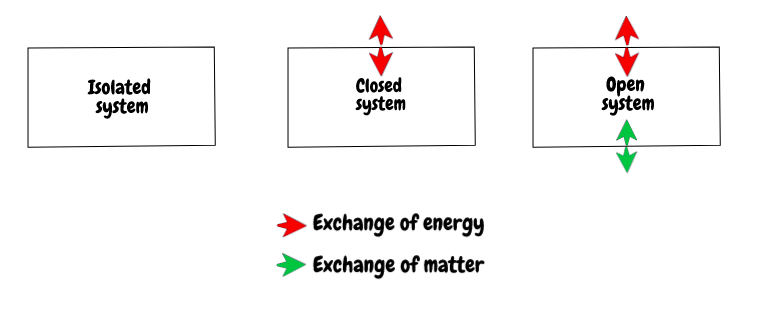
So there we go, a concept based approach to help students construct their own understanding of isolated, closed and open systems in chemistry. I would love to hear your thoughts and if you have any examples of concept-based approaches to teaching in your classroom.
Teaching like this has the potential to completely flip science education. This example in particular relates to physics and chem explicitly and could definitely be used to really get students to think about what that terminology means and how it applies in those different situations. Our method of teaching chemistry through topics definitely has a "story" and a build up of skills but sometimes there's just so much content that means cementing those conceptual understandings and drawing inter-disciplinary links doesn't happen as much as we'd like. It becomes "extension work" when it should really be the central idea.... work in progress right?