Some of the terminology that we use in chemistry can be extremely confusing for students! One such example is when we talk about bonding.
A bond is the name given when electrostatic forces of attraction between oppositely charged particles are balanced with the kinetic energy that would cause them to move away from each other in random directions. Put more simply:
“A bond is an electrostatic force of attraction between oppositely charged particles”.
Within chemistry, this is an extremely important idea. When approaching this topic, most literature will talk about intramolecular and intermolecular bonding. Confusingly, intramolecular bonding (meaning within molecules) will include ionic bonding alongside covalent bonding (including simple molecules and giant covalent structures). However, there are no molecules in giant ionic lattices. This is a breeding ground for misconceptions!
Intermolecular bonding (meaning between molecules) works much better as we only include temporary dipoles, such as London Dispersion Forces (LDF) and permanent dipoles, including hydrogen bonding. All the bonds encompassed here are between molecules, which makes perfect sense. Phew!
Beside the problem with ionic boning residing under the term intramolecular bonding, the whole system also completely ignores the electrostatic force of attraction between electrons and protons within an atom! This is incredibly important and students often cannot link this idea with the other concepts in bonding.
I therefore propose a new way of naming bonds that hopefully overcomes these challenges. This is summed up in figure 1. Unfortunately, you will need to zoom in at this point in order to see it fully, but hopefully, you can get the idea. The key features of this diagram are:
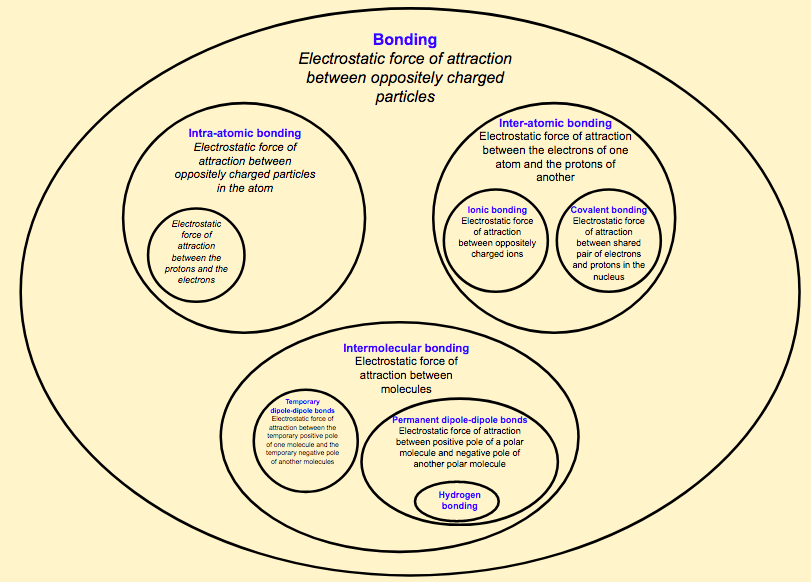
Figure 1 - A new naming system for bonding - what do you think?
How I would teach this is a matter for another blog post! In the meantime, I would love some feedback on this taxonomy.
Firstly, you will notice the glaring omission of metallic bonding. Where might this fit in?
Secondly, in more general terms: What do you think of this new naming? Is there anything I have missed? Are any of my definitions wrong? Am I completely off the mark? How might you use it in your teaching?