There are certain concepts in chemistry that students must understand if there are to develop an expertise in the discipline. We can call these “threshold concepts”. These threshold concepts should be considered as a portal to further understanding and, according to Meyer and Land (2003), should be:
- Transformative - once understood, it should cause a significant shift in the thinking of the students. Chemistry example: when students come to understand matter is composed of atoms.
- Probably irreversible - the change in perspective is unlikely to be forgotten or can only be unlearned with significant effort (this has implications when students learn a misconception). Chemistry example: once students understand that both forward and backward reactions can occur, it is very difficult to tell them that reactions go to completion.
- Integrative - understanding the threshold concept exposes a previously hidden interrelatedness of something. I liken this to the notion of linking two concepts to form a conceptual understanding in the concept based teaching advocated by Lynn Erickson and Lois Lanning. Chemistry example: understanding that all particles have kinetic energy helps students to link the idea of particles colliding to the energy required to break bonds.
- Bounded (athough not always) with a conceptual space that has terminal frontiers. The concept eventually reaches a border which, when stepped across, leads into a new threshold concept or even a new discipline. Chemistry example: the threshold concept of atomicity within the discipline of chemistry is not easily applied to the discipline of history!
- Troublesome - they are problematic and difficult for students to understand. They must challenge themselves to enter the space of uncertainty that lies between their current understanding and a higher understanding. Chemistry example: The concept of wave particle duality is incredibly troublesome for students. To conceive of a particle as a wave requires some incredibly abstract thinking, which is hard for students to achieve.
Identifying these threshold concepts is not easy, but working through the checklist below might be helpful. I will be looking to identify the threshold concepts in chemistry in an upcoming blog post, but I have made a start here, (hmmmm, would all the concepts I identified in this post pass the checklist below...?) as has my colleague, Oliver Canning, here. In the meantime, I would love to hear some thoughts on the following questions:
- How do you like the checklist for identifying threshold concepts? What would you change, add or take away?
- What do you think the threshold concepts in chemistry are and why?
- Have I misinterpreted anything or got anything wrong? I would love to know!
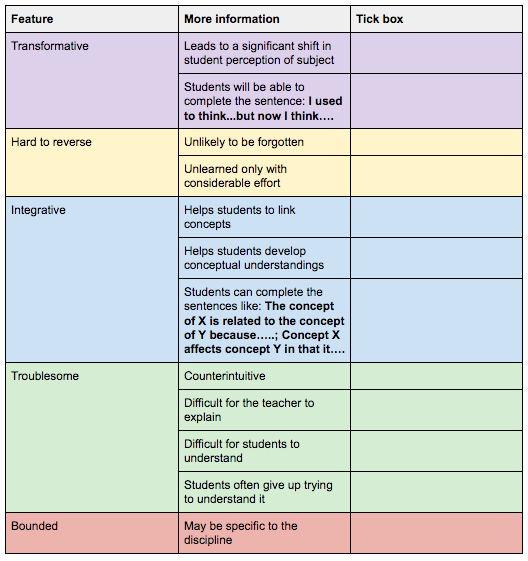
Figure 1 - a potential checklist for identifying threshold concepts
Further reading
Meyer, J.H.F. and Land, R. (2003), ‘Threshold concepts and troublesome knowledge (1): linkages to ways of thinking and practising’, in Rust, C. (ed.), Improving Student Learning – ten years on, Oxford: OCSLD: https://www.dkit.ie/system/files/Threshold_Concepts__and_Troublesome_Knowledge_by_Professor_Ray_Land_0.pdf

[…] recently wrote about threshold concepts in this post. As stated in the article, threshold concepts are often troublesome for learners. However, they […]