Pre-warning. This is a bit of a long read and gets a bit heavy. If you want to skip to the final summary, scroll down! Disclaimer: A lot of this article is based on the work of Talanquer (2015). Barely any of it is my own so please do take a look at his article for the original work.
As discussed in this post, ontological components make up part of the complex cognitive process that help students to access an understanding of threshold concepts. The ontological component involves learners developing schema that help them to think about the nature of the entities and processes present in the systems that they are studying. Further, it involves them thinking about the nature of the relationships that exist between these entities and processes.
Talanquer (2015) outlines five internal schema that students of chemistry often have which might limit their understanding and ability to develop expertise in the subject. He then goes on to outline some internal schema that we might help students develop so that they are better able to develop said expertise. In order to move from these ‘limiting’ internal schema to the more ‘adaptive’ internal schema, we might scaffold the experiences of the students so that they can uncover conceptual understandings that help them ‘see’ the chemistry world in a more flexible manner.
The five ‘limiting’ internal schema are outlined in the diagram below, along with the more ‘adaptive’ internal schema that we hope students of chemistry will attain. You can see that the key to unlocking the more powerful schema could be carefully thought out conceptual understandings. Some ideas for some conceptual understandings that will aid this transition are suggested in the coming sections of this article.
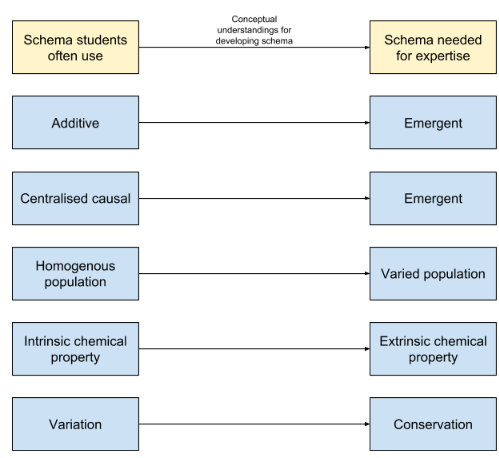
From the additive property to the emergent internal schema
The problem with students seeing chemistry with an additive property scheme is that they think the everything in chemistry is the sum of its parts. In this schema, if you add one atom to another, it has twice as much mass, twice as much energy, twice as much everything! Another example might be a student thinking that more energy is required to break the bonds of a molecule with more bonds. However, we know that this is not necessarily the case (think about double bonds vs single bonds). In this schema, particles have a known, concrete state and are not dynamic in nature. They are not affected by time and space.
Instead, it is suggested that students develop an emergent internal schema. Here, students recognise that systems in chemistry are dynamic, sensitive to time and space. They also recognise that measurable properties are average values of targeted quantities (e.g., number of particles per unit volume, kinetic energy per particle) over the many configurations of the measurements timespan (Talanquer, 2015).
Some conceptual understandings to move students from the additive property to the emergent internal schema might be:
From the centralised causal to the emergent internal schema
In the centralised causal schema, students view particles as protagonists. Fluorine wants to gain an electron so it can be happy and get a full outer shell. An acid donates a proton to a base so it can become more stable. In this schema, particles are often deemed as having goals that should be met in order to reach a more desirable state. According to Talanquer (2015),
“Highly reactive substances are commonly seen as the initiators in chemical reactions, and changes in the properties of a solution are often attributed to the active action of solute particles on solvent particles”.
This idea of protagonists and causation is embedded in everyday human life. Therefore, it is not surprising that students come to visualise chemistry in this way. It is very comforting and indeed, I often find myself falling into this schema. It is often the easiest (dare I say, laziest…) way to explain something in chemistry.
However, we know that observable properties at the macroscopic domain are due to dynamic, random and continuous interactions of particles at the microdomain. There are a multitude of random interactions occurring at the same time throughout a chemical system and the macroscopic outcomes of these interactions depends on relative probability of random events occurring, and this depends on internal and external constraints placed upon the system. For example, fluorine can quite happily exist as an atom with an unpaired electron if it is given enough energy. It doesn’t ‘want’ to gain an electron to become more stable. It is just that in certain situation, the external and internal pressures on fluorine will lead it to do so.
So, some conceptual understandings to move students from the centralised causal schema to the emergent internal schema might be:
From the homogenous population to the varied population schema
Personally, I think this is one of the most important realisations that a student can make. Students who see chemistry through the homogenous population schema think of all the particles of the same type as identical, rigid objects moving at the same speeds (Talanquer, 2015). This understanding might stem from a lack of understanding of what a chemical equation is representing. For example, in the equation A + B → AB , students often think of a single atom of A reacting with a single atom of B to give a single molecule of AB. In this schema, students would think that all particles of A and all particles of B will turn into AB at the same time.
However, particles of the same type in a substance are not all the same. A very simple example is that of kinetic energy. We know that particles of the same type in a substance may have different kinetic energies. This affects their ability to react when colliding with other particles. In the above example of A + B → AB, this means that not all of A can react with B at the same time! Hence why we use temperature as a measure of average kinetic energy. In a substance, there are constant variations and interactions occurring that mean that not all the particles are the same. Further, students should understand that chemical and physical changes that seem to follow a clear direction at the macroscopic level are due to random fluctuations in the spatial and energetic distribution of particles at the microscopic level. Without developing this internal schema, it might be very hard for students to grasp fundamental concepts such as equilibrium.
So, some conceptual understandings to move students from the homogenous population schema to the varied population internal schema might be:
From the intrinsic chemical property to the extrinsic chemical property schema
This was a bit of a revelation for me. In the intrinsic chemical property schema, learners will often see the chemical properties of a substance as an intrinsic characteristic that determines its behavior under all conditions (Talanquer, 2015). For example, they might expect a strong acid to be strong in all situations. However, should hydrochloric acid be thought of as a strong acid when placed in non-aqueous conditions? The answer, I think, is no. Therefore, it is not the intrinsic properties of hydrochloric acid that define it as a strong acid. It is instead the impact of external particles and conditions, such as the presence of water molecules. Indeed, the chemical properties of a substance are actually caused by the environment in which they are placed. They are extrinsic in nature.
When adopting the extrinsic chemical property schema, learners are able to think of chemical properties as relative and dependant on the nature of interaction systems. A student who does not understand this might have a hard time understanding that water can act as a base when placed in the same environment as hydrochloric acid, but as an acid when placed in the same environment as ammonia!
To help scaffold students transition to an extrinsic property schema, we might use the following conceptual understandings:
From the variation to conservation schema
I have a confession to make here. I struggle to understand this one fully. However, I will give it a go. Talanquer (2015) cites studies that suggest student reasoning is highly constrained by explicit clues, such as a change in physical appearance. As far as I read it, this means that students tend to focus on the changes that occur, and that this constrains the level of understanding that they can reach. It is human nature to detect changes in our environment and we are very good at it. We feel an urge to try and explain what causes these changes, but often pay little attention to explain what we perceive as the natural state of things. It takes individuals with astounding curiosity to ask the questions about the constants in the world around us. But, this is an issue, as chemists who have the aim of trying to explain, predict and control changes in the material, macroscopic domain often look to identify what is conserved during a process. This exemplified by fundamental principles like conservation of mass and energy. Focusing on these constants helps them find relationships between a systems components, and in turn allows them to think of ways to manipulate other systems. They recognize and exploit the constancy over time and space of properties of a reacting mixture (e.g., equilibrium constants, chemical potentials) to build predictive models. In order to adopt this schema of conservation, students must focus on the implicit, rather than the explicit (change in appearance) of chemical systems.
Some conceptual understandings that might (emphasis on the might) be of use here are:
In summary
If you have made it this far, then I applaud you. The following table gives a short summary of this post and I hope you find it useful. I would love to hear some thoughts on this post. A lot is up for debate and I am not sure I have fully understood anything!

References
Talanquer, 2015: https://pubs.acs.org/doi/pdf/10.1021/ed500679k
[…] Ontological components – according to Talanquer, this involves learners developing proper schemas that help them to think about the nature of the entities and processes in the systems under consideration and going even further, the relationships that exist between them. Chemistry example: again from the threshold concept of atomicity, Talanquer suggests that the statement “matter consists of atoms that have internal structures that dictate their chemical and physical behavior” will look very different to a student who views the atom as a solid object with rigid internal structures they will think very versus a student who views atoms as dynamic, interacting systems. This area is a major challenge and is the focus of this post. […]